Insight
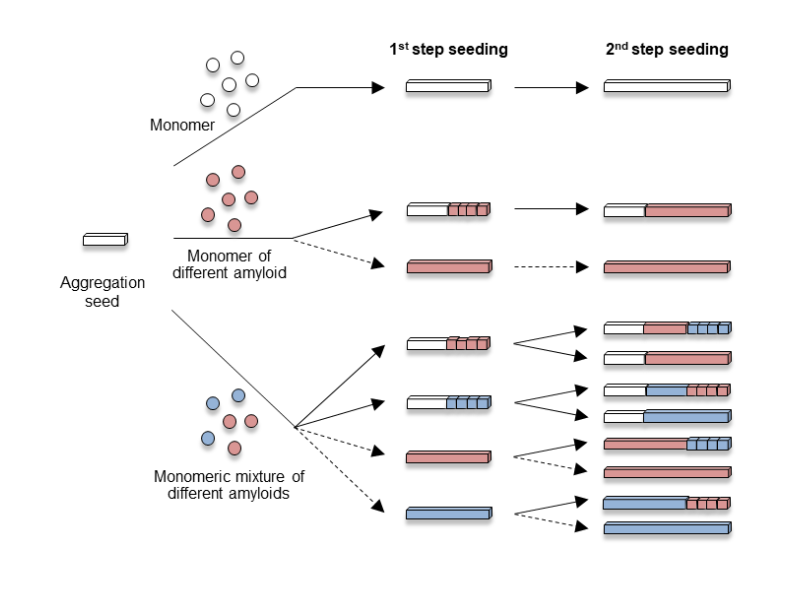
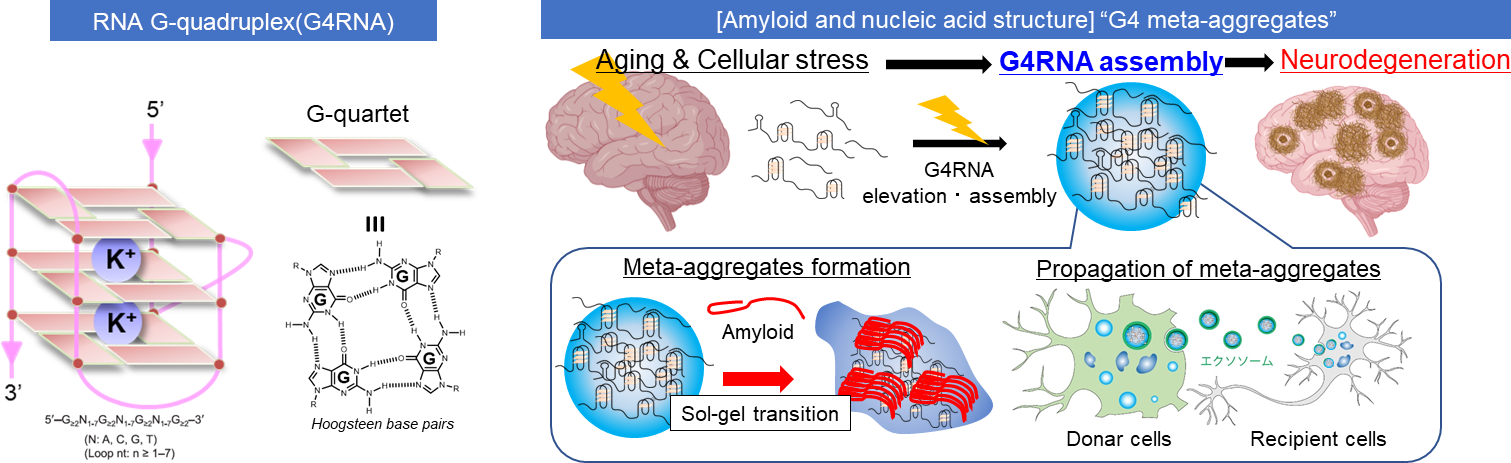
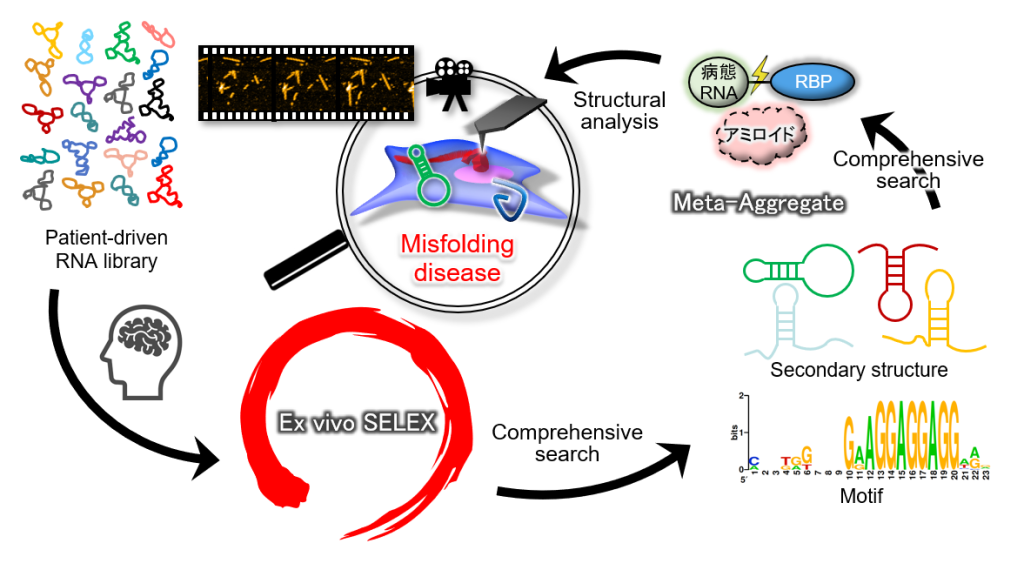
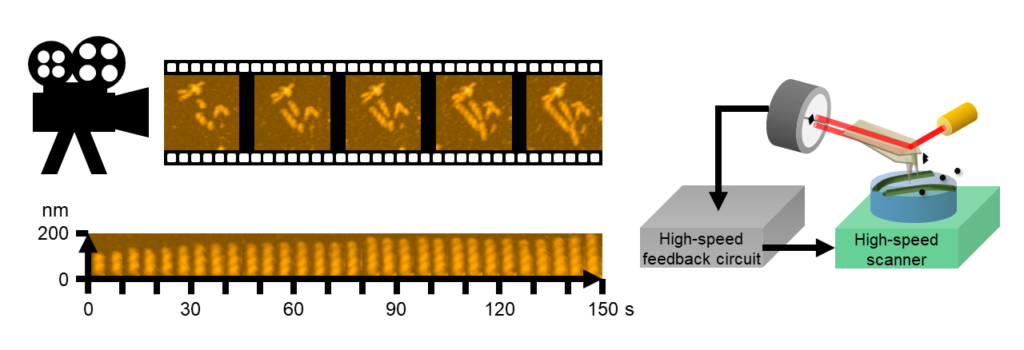
In misfolding diseases, amyloidogenic proteins form large aggregates, which can be toxic to neuronal cells, leading to the disease state. In vitro experiments have shown that amyloid aggregates with other amyloids of the same type. However, recent discoveries in neurodegeneration and aging have revealed that amyloid can also co-aggregate with biomolecules present in high concentrations in the cytosols. Therefore, it is crucial to adopt a panoramic perspective (MetaView) and examine mixed states (MetaStable), where various forms of aggregates combine with different biomolecules. We refer to these aggregates as "MetaAggregates," and our research aims to understand how they are formed. Once this process is clarified, we will use it as a blueprint to develop inhibitors with minimal adverse effects, focusing on small molecules and nucleic acid medicines.